Understanding US20120065539A1: An Innovative Approach to Breast-Mass Detection Using Impedance Techniques
US20120065539A1 represents a groundbreaking development in the realm of non-invasive breast cancer diagnostics. This patent introduces a method that utilizes impedance-based technology to detect and characterize breast masses with enhanced precision and safety.
Traditional imaging tools like mammography and ultrasound have long dominated the diagnostic space, but US20120065539A1 challenges their limitations with a unique, affordable, and non-radiative alternative.
The driving force behind this invention is the need for early detection methods that are not only accurate but also accessible and comfortable for patients. With a growing emphasis on preventive healthcare, technologies like the one proposed in US20120065539A1 are essential in bridging gaps in diagnostic coverage—especially in low-resource settings.
Technical Field
The technology covered under US20120065539A1 is rooted in biomedical impedance analysis. Specifically, it applies to detecting anomalies in breast tissue by measuring how electrical signals interact with different tissue types. This patent not only focuses on breast masses but also hints at broader medical potential. Similar impedance-based mechanisms could be adapted to assess organs like the liver or lungs, extending its utility across multiple diagnostic platforms.
Summary of the Invention
At its core, US20120065539A1 outlines a device and method capable of detecting variations in electrical impedance caused by abnormalities such as tumors or cysts. The system sends a low-energy electrical signal through the breast tissue and records how the signal is altered. These changes are then compared to known impedance profiles, allowing the system to identify potential masses.
Objectives of the Invention:
- Offer a non-invasive, pain-free alternative to mammography
- Improve diagnostic accuracy using real-time impedance mapping
- Enable point-of-care testing in both clinical and remote environments
Detailed Description
The patent goes into meticulous detail about the construction and functionality of the system. Below is a breakdown of the key components and how they work together to perform breast-mass detection.
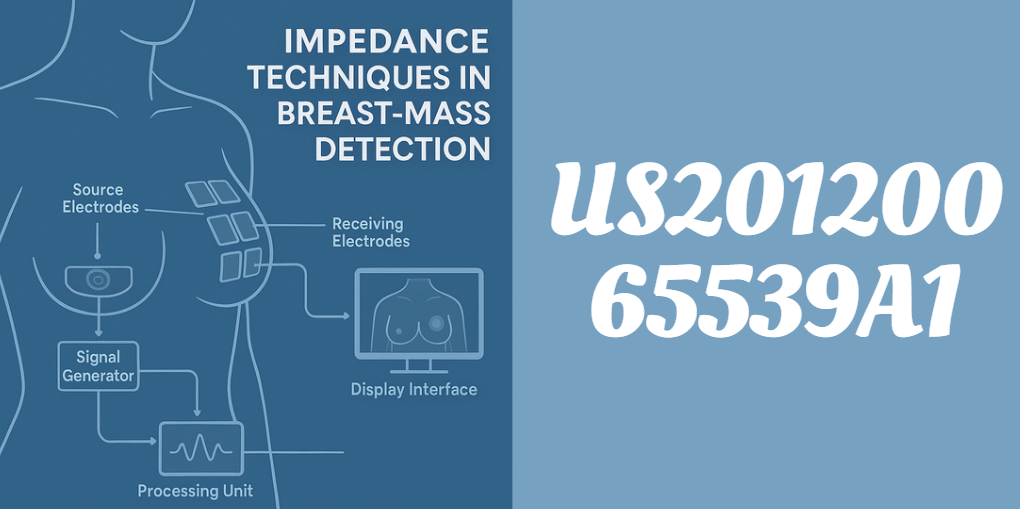
System Components
| Component | Functionality |
|---|---|
| Signal Generator | Produces electrical signals at various frequencies |
| Source Electrodes | Apply signals to the tissue |
| Receiving Electrodes | Capture altered signals from the tissue |
| Processing Unit | Analyzes signal alterations using software algorithms |
| Display Interface | Presents diagnostic feedback to the user through visual or audible cues |
The electrodes can be arranged in various configurations depending on the area to be examined. The patent discusses both fixed and dynamic placements, ensuring flexibility during use.
Signal Characteristics
Signals used in this system vary in frequency and waveform. For effective tissue discrimination:
- Low-frequency signals penetrate deeper tissues
- High-frequency signals enhance surface resolution
Together, these enable the detection of both superficial and embedded masses.
Data Analysis and Interpretation
The collected signals are subjected to digital signal processing (DSP), which helps extract meaningful patterns from the noise. Algorithms compare this data against a database of known impedance signatures. The outcome can include:
- Identification of mass location
- Classification as benign or suspicious
- Recommendations for further testing
Methodology
The procedural flow of US20120065539A1’s method is as follows:
- Electrode Placement: Electrodes are applied to the breast, ensuring optimal contact.
- Signal Emission: The generator sends a predefined range of frequencies through the tissue.
- Signal Reception: Receiving electrodes collect the altered waveforms.
- Digital Analysis: The system evaluates amplitude, phase shifts, and waveform patterns.
- Comparison: The results are compared with stored impedance data of healthy and abnormal tissues.
- Diagnosis Output: The final analysis is provided via display or external connection to diagnostic tools.
This step-by-step process ensures accuracy and reproducibility, making the system suitable for both clinical and home settings.
Clinical Applications
US20120065539A1 is not merely a theoretical concept—it is designed for practical use across multiple healthcare domains:
Key Use Cases:
- Routine Screening: An alternative for individuals unable or unwilling to undergo mammograms.
- Triage in Remote Clinics: Its portability makes it ideal for use in under-resourced regions.
- Post-treatment Monitoring: Helps track changes in tissue composition over time.
- Adjunct to Imaging: Can be used alongside ultrasound or MRI for a more complete picture.
Comparative Analysis
When measured against existing diagnostic tools, US20120065539A1 holds several advantages:
| Diagnostic Tool | Radiation Exposure | Portability | Real-Time Results | Cost |
|---|---|---|---|---|
| Mammography | Yes | Low | No | High |
| Ultrasound | No | Moderate | Yes | Moderate |
| MRI | No | Low | No | Very High |
| US20120065539A1 | No | High | Yes | Low |
Advantages Over Existing Methods
The patented method offers numerous benefits:
- Non-Invasive: No needles, radiation, or compression involved.
- Cost-Effective: Uses low-cost components and requires minimal training.
- Patient-Friendly: Ideal for sensitive populations such as pregnant women or those with implants.
- Versatile: Can be integrated into handheld devices or larger diagnostic systems.
Potential for Expansion
While breast-mass detection is the primary focus, US20120065539A1 hints at a broader scope. Because the underlying principle—impedance variation—is common across biological tissues, the technology could be adapted for:
- Liver fibrosis assessment
- Prostate mass detection
- Muscle tissue evaluation in sports medicine
- Lung function tests for respiratory illnesses
Conclusion
US20120065539A1 is more than just a patent—it’s a window into the future of accessible, accurate, and patient-centered diagnostics. Its use of impedance techniques offers a revolutionary pathway to identifying breast masses without the downsides of traditional methods. Whether used in hospitals, clinics, or mobile units, this innovation is poised to redefine how we approach early cancer detection.
As healthcare trends toward personalization and decentralization, patents like US20120065539A1 illuminate the path forward. It’s not just about detecting disease—it’s about doing so intelligently, affordably, and with compassion.
Recommended Articles
Munchabilies THC Trippy: The Ultimate Guide to Psychedelic Cannabis Edibles
Durostechcom: Revolutionizing the Future of Technology
Spring Ferraz A-102914 Right: Complete Guide to Specifications, Uses & Maintenance
Morjier255: The Ultimate Guide to Ecological Monitoring & Productivity Management